Have you ever wondered how scientists and engineers meticulously measure the world around us? From the tiniest molecule to the vast expanse of the cosmos, accurate measurement is the foundation of our understanding. And in the heart of this measurement system lies a critical component: units. Today, we’re embarking on a journey to explore the fascinating world of 2.4.4 journal measurement and units, unraveling the key to accurately interpreting and applying the scientific discoveries published in esteemed journals.
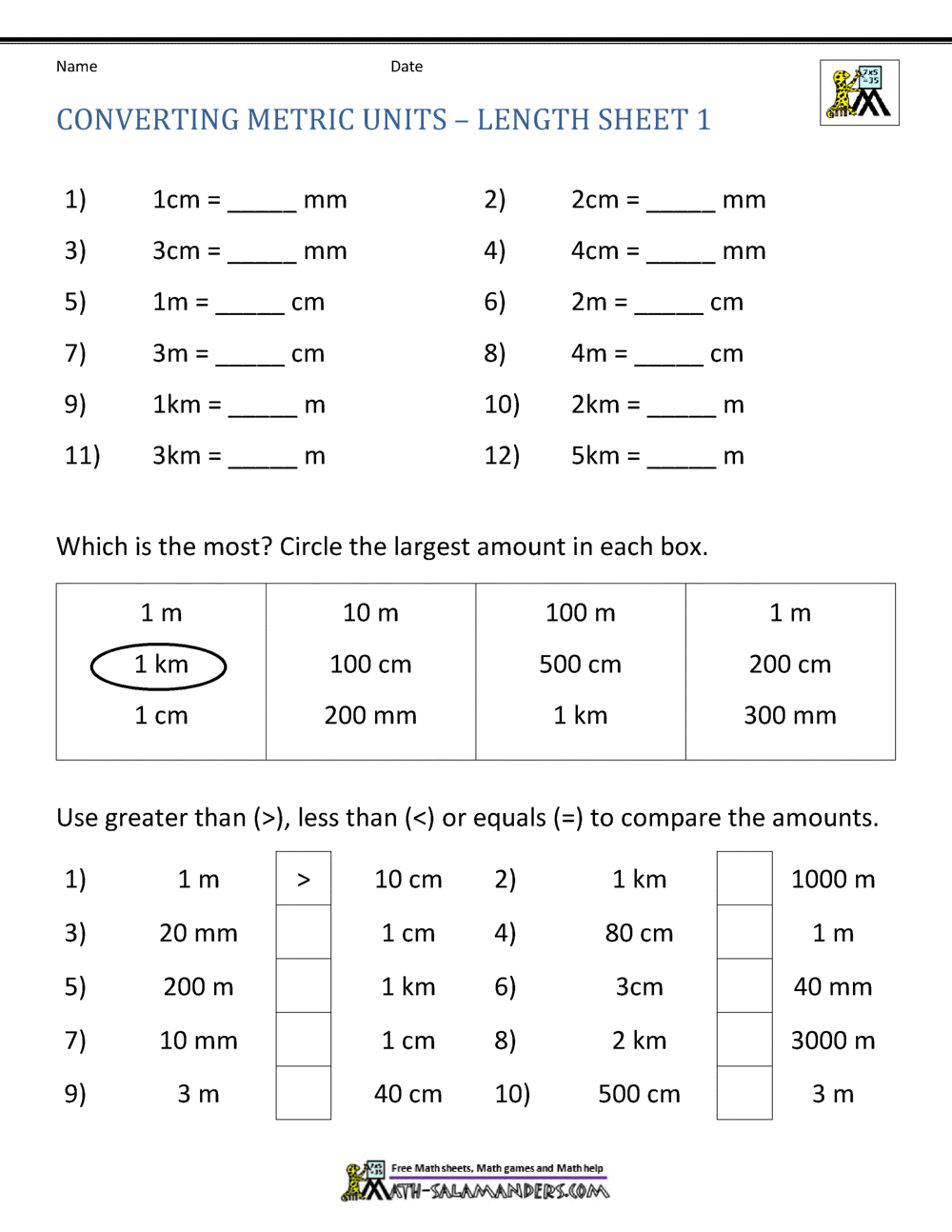
Image: classfullchristina.z19.web.core.windows.net
The 2.4.4 journal measurement and units system isn’t just about numbers; it’s a language that allows scientists to communicate their findings clearly and precisely. Think of it as a universal translator ensuring that researchers across the globe can understand and build upon each other’s work. In this guide, we’ll delve into the history, concepts, and real-world implications of this essential framework.
Understanding the Foundation: A History of Measurement Units
From Ancient Times to Modern Standards
The quest for measurement dates back to ancient civilizations. Egyptians, for instance, used cubits, based on the length of a forearm, to build their magnificent pyramids. The Greeks and Romans refined these early systems, introducing concepts like the foot and the mile. However, these systems lacked standardization, leading to inconsistencies and confusion.
The Birth of the Metric System
The 18th century saw a pivotal moment in the history of measurement – the emergence of the metric system. This system, based on decimal units, promised a unified and universally applicable framework. The core unit of length, the meter, was defined as one ten-millionth of the distance from the North Pole to the equator. The metric system revolutionized measurement, creating a more efficient and practical system for science, trade, and everyday life.
.jpg)
Image: betrained.in
The International System of Units (SI)
As scientific knowledge expanded, the need for a more comprehensive and standardized system emerged. In 1960, the International System of Units (SI), built upon the metric system, was adopted by international organizations, solidifying its global influence.
Deciphering the Language of Measurement: 2.4.4 Units
The Importance of Units
Imagine a recipe that tells you to add “a bit of salt.” This ambiguity would lead to disastrous results! Just like a recipe, scientific measurements require precise units to be meaningful. Units provide context, allowing us to interpret numbers accurately. For example, 10 kilograms of sugar is vastly different from 10 grams of sugar.
The 2.4.4 System: A Guide for Scientific Communication
The 2.4.4 system, often referenced in scientific journals, provides a standardized framework for units. It specifies the appropriate unit for each type of measurement, eliminating ambiguity and ensuring consistency. Let’s break down the key elements of 2.4.4:
- Length: The meter (m) is the basic unit of length in the 2.4.4 system. For smaller measurements, centimeters (cm) or millimeters (mm) are used, while kilometers (km) are used for larger distances.
- Mass: The kilogram (kg) is the standard unit of mass. Grams (g) and milligrams (mg) are used for smaller masses.
- Time: The second (s) reigns supreme for measuring time intervals. Minutes (min) and hours (h) are also commonly used.
- Temperature: The Kelvin (K) is the absolute temperature scale, often used in scientific contexts. The Celsius (°C) scale is more familiar for everyday measurements.
- Volume: The liter (L) is the basic unit for volume. Milliliters (mL) are commonly used for smaller volumes.
Bridging the Gap: Real-World Applications of 2.4.4 Units
The Impact on Scientific Advancements
2.4.4 units are not just abstract concepts; they are integral to our daily lives. From the medical devices that diagnose diseases to the airplanes that soar through the skies, accurate measurement is at the core of technological innovation.
Examples in Action
- Pharmaceuticals: Dosing medications relies heavily on precise measurements. A drug’s effectiveness depends on the accurate amount administered to the patient, measured in milligrams or micrograms.
- Engineering: In the construction of bridges and buildings, every measurement needs to be exact. Millimeters of error can lead to significant structural weaknesses.
- Environmental Monitoring: Scientists use 2.4.4 units to measure pollution levels, track climate change, and understand the health of ecosystems. Accurate measurements are critical for environmental protection and sustainable development.
The Future of Measurement: Staying Ahead of the Curve
Advancements in Measurement Technology
The world of measurement is constantly evolving. New technologies, such as laser interferometry and atomic clocks, are pushing the limits of precision. Scientists are now capable of measuring quantities with unprecedented accuracy. These advancements are driving scientific discovery and leading to transformative innovations.
The Role of 2.4.4 in the Future
As we journey into the future of science and technology, 2.4.4 units will remain an indispensable cornerstone. Their standardization ensures the reliability and reproducibility of scientific experiments, paving the way for groundbreaking discoveries.
2.4.4 Journal Measurement And Units Answer Key
Conclusion: The Power of Precision
The 2.4.4 journal measurement and units system is a testament to human ingenuity. Through standardized units, scientists can communicate and collaborate effectively, building upon each other’s work to unravel the mysteries of the universe. Whether you’re a researcher, a student, or simply someone intrigued by science, understanding this critical framework unlocks a deeper appreciation for the world around us. So, let’s embrace the power of precision, allowing us to measure, understand, and shape the world to its fullest potential.






