As a passionate chemistry enthusiast, I’ve always been fascinated by the intricacies of the elements that make up our universe. One element that particularly piqued my curiosity is lithium, a soft, silvery-white metal renowned for its unique properties. I remember being introduced to lithium’s electron configuration in my high school chemistry class – a captivating concept that opened a door to understanding its behavior and applications. Today, we’ll delve into the fascinating world of Li electron configuration, exploring what it is, why it matters, and what makes this seemingly simple element so extraordinary.

Image:
Understanding the arrangement of electrons in an atom’s shell is fundamental to comprehending its chemical behavior. This arrangement, known as the electron configuration, is like a blueprint revealing how an atom interacts with other atoms to form molecules and participate in chemical reactions.
Understanding Li Electron Configuration: A Glimpse into Lithium’s World
Lithium, with its atomic number 3, occupies a unique position in the periodic table as the lightest alkali metal. The Li electron configuration, [He] 2s1, reveals that it possesses a total of three electrons. Two of these electrons fill the 1s orbital, mimicking the configuration of helium, a noble gas known for its stability. The remaining electron resides in the 2s orbital, making lithium eager to shed this lone electron in pursuit of greater stability.
The Li electron configuration explains why lithium is so reactive, readily losing this lone outer electron. This tendency to lose one electron to achieve a complete outer shell, like helium, is the very reason lithium forms cations with a +1 charge. This characteristic also makes lithium an excellent conductor of electricity, a property that finds applications in batteries and other electrical components.
Key Concepts: Delving Deeper
To gain a more profound grasp of Li electron configuration, let’s dissect its components:
- [He]: This notation signifies the electronic configuration of helium, 1s2. It represents the filled inner shell of lithium, symbolizing stability.
- 2s1: This part indicates that the remaining electron resides in the 2s orbital. The superscript ‘1’ denotes that only one electron occupies this orbital.
Understanding the Li electron configuration helps us understand how lithium behaves in chemical reactions. It readily forms ionic bonds by losing its lone valence electron in the 2s orbital, striving to achieve a stable, helium-like configuration. This tendency to readily lose an electron explains why lithium forms ionic compounds like lithium chloride (LiCl), lithium oxide (Li2O), and lithium hydroxide (LiOH). These compounds are essential for various applications in different fields.
The Li electron configuration also sheds light on lithium’s unique properties. Its low ionization energy, meaning it takes relatively little energy to remove an electron, makes it an excellent reducing agent. Lithium’s small ionic radius and high charge density explain why it’s a powerful reagent used in organic synthesis.
Applications of Li Electron Configuration in Modern World
The knowledge gained from Li electron configuration has led to a wide range of applications, revolutionizing various fields:
- Batteries: Lithium-ion batteries, renowned for their high energy density and long lifespan, dominate the portable electronics and electric vehicle industries. The Li electron configuration is crucial in understanding the battery’s charge and discharge processes, involving lithium ions moving between electrodes.
- Medicinal Chemistry: Lithium salts have found therapeutic applications in managing mood disorders like bipolar disorder. While the precise mechanism of action is not fully understood, researchers believe lithium ions interact with signaling pathways within neurons, influencing brain chemistry.
- Organic Chemistry: The Li electron configuration fuels a wide array of reactions in organic synthesis. Lithium reagents such as lithium aluminum hydride (LiAlH4) and lithium diisopropylamide (LDA) act as powerful reducing agents and strong bases, facilitating the creation of various organic compounds.
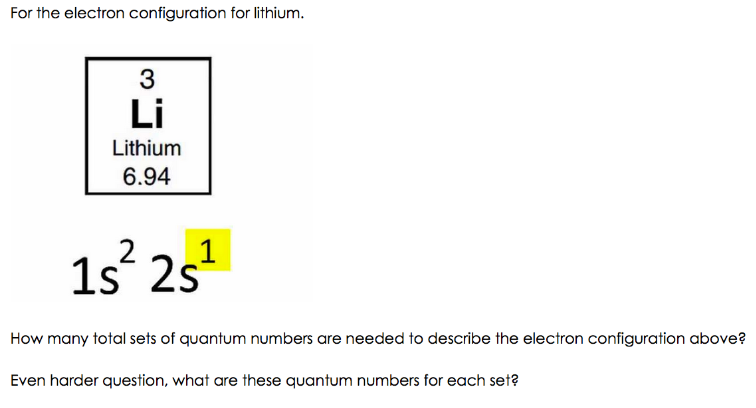
Image:
Tips and Expert Advice
When exploring Li electron configuration, it’s crucial to visualize the arrangement of electrons within the atomic orbitals. Employ diagrams like orbital diagrams and electron configuration notation to enhance understanding. Practice writing electron configurations for other elements, and keep the periodic table handy as a reference.
Embrace online resources like Khan Academy, YouTube videos, and interactive simulations to grasp the intricacies of electron configuration. Don’t hesitate to seek help from your instructor, classmates, or online communities to clear any doubts or challenges you face.
Frequently Asked Questions (FAQ)
What is the electronic configuration of lithium?
The electronic configuration of lithium is [He] 2s1. It has three electrons; two fill the 1s orbital, mimicking helium, and the third resides in the 2s orbital.
Why is lithium reactive?
Lithium is reactive because it has a lone valence electron in the 2s orbital. It strives to lose this electron, achieving a stable, helium-like configuration. This tendency to readily lose an electron makes it highly reactive, prone to forming ionic bonds.
What are the uses of lithium?
Lithium finds widespread applications in various fields. It’s a key component in lithium-ion batteries, essential for portable electronics and electric vehicles. Additionally, lithium salts have therapeutic uses in managing mood disorders, and lithium reagents play a vital role in organic synthesis.
Li Electron Configuration
Conclusion
Lithium’s electron configuration, [He] 2s1, is the key to understanding its unique properties and diverse applications. From powering our gadgets to influencing brain chemistry, this seemingly simple element plays a crucial role in our modern world. By comprehending Li electron configuration and its implications, we unlock the secrets of this enigmatic and vital element.
Are you fascinated by the world of chemistry and the role of Li electron configuration? I encourage you to explore further, delve deeper, and share your thoughts and discoveries in the comments below. Let’s continue to unravel the mysteries of the elements that shape our universe.






