Have you ever wondered why a rusting nail, a glowing lightbulb, and a delicious slice of toast all share a common thread? The answer lies in the fascinating realm of chemistry, where atoms engage in a constant dance of electron exchange. This dynamic interplay is quantified by a crucial concept known as the oxidation number—a powerful tool that helps us understand and predict chemical reactions. But navigating the intricacies of oxidation numbers can be daunting, especially for those new to the subject. Fear not! This comprehensive guide will demystify oxidation numbers and equip you with the knowledge to use an oxidation number calculator like a pro.
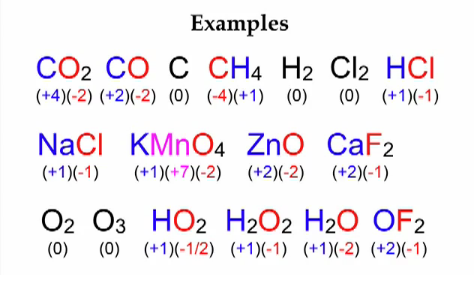
Image: www.w3schools.blog
At its core, the oxidation number is a numerical value that represents the hypothetical charge an atom would have if all its bonds were entirely ionic. This might seem abstract at first, but understanding its implications is key to mastering chemistry. By knowing the oxidation number of an atom within a molecule or ion, we can decipher its role in a reaction, predict the types of bonds it forms, and even forecast the products of those reactions. But how do we determine these oxidation numbers without getting entangled in complex equations? Enter the oxidation number calculator, a digital companion that effortlessly unveils the hidden secrets of atomic charge.
Decoding the Oxidation Number: A Journey into the Heart of Chemistry
To truly appreciate the power of an oxidation number calculator, let’s delve into the history of this concept. The notion of oxidation numbers arose from the pioneering work of chemists like Humphry Davy and Jöns Jacob Berzelius in the early 19th century. Their discoveries revealed that many chemical reactions involve the transfer of electrons, leading to changes in the electric charge of atoms. Over time, chemists developed a standardized system for representing these changes, ultimately giving birth to the concept of the oxidation number. It’s a bit like a secret code, where each element’s numerical signature tells us its role in a chemical symphony.
Understanding oxidation numbers requires grasping a few fundamental principles. First, the sum of the oxidation numbers of all atoms in a neutral molecule is always zero. For an ion, the sum of the oxidation numbers equals the charge of the ion. Second, certain elements consistently exhibit predictable oxidation numbers, acting as reliable benchmarks within the periodic table. For instance, metals in their elemental state have an oxidation number of zero, while alkali metals (Group 1) typically have +1, and halogens (Group 17) usually have -1. Understanding these basic rules equips you with a solid foundation to tackle more complex scenarios.
Navigating the Labyrinth: The Practical Power of an Oxidation Number Calculator
Imagine you’re presented with a complex chemical compound, say, potassium permanganate (KMnO4). Determining the oxidation number of each element in this compound manually can be a lengthy and intricate process. This is where an oxidation number calculator steps in, effortlessly deciphering the atomic charges and revealing the chemical dance happening within the molecule. By inputting the chemical formula, the calculator utilizes a predefined set of rules and algorithms to assign oxidation numbers to each element, eliminating the need for complex calculations and ensuring accuracy.
Using an oxidation number calculator offers numerous advantages:
- Effortless Solutions: Calculators rapidly provide oxidation numbers for any molecule or ion, freeing up your time for deeper analysis.
- Enhanced Accuracy: The calculators are programmed with precise rules and algorithms, reducing the risk of human errors.
- Versatile Applications: Whether you’re studying basic chemistry, researching chemical reactions, or even analyzing complex biological processes, oxidation number calculators are invaluable tools.
- Learning Aid: By visualizing the results of different compounds, you can gain a deeper understanding of the factors influencing oxidation numbers.
Beyond the Numbers: Understanding the Significance of Oxidation Numbers
While the calculator provides the numerical answer, understanding the underlying principles behind oxidation numbers is paramount. Each oxidation state represents a unique charge distribution within a molecule, dictating how atoms interact and react. Oxidation numbers underpin essential chemical concepts like redox reactions, where electrons are exchanged between species. This electron transfer forms the basis of many crucial processes, including:
- **Respiration:** The process by which organisms extract energy from food, involving the transfer of electrons through a series of reactions.
- **Corrosion:** The deterioration of metals due to chemical reactions with their environment, driven by electron transfer.
- **Electrochemistry:** The study of the relationship between chemical reactions and electrical energy, where oxidation and reduction processes generate electric currents.
- **Batteries:** The energy storage devices that rely on redox reactions to convert chemical energy into electrical energy.
Understanding oxidation numbers provides us with a window into the intricate dance of electrons within chemical processes. It empowers us to predict reactions, control reactions, and even harness the power of electron transfer for various applications.

Image: alexiayouthkirk.blogspot.com
Expert Insights and Actionable Tips for Mastering Oxidation Numbers
To further enhance your understanding of oxidation numbers, here are some insights from seasoned chemists and actionable tips for applying this knowledge:
- Embrace the Periodic Table: Familiarize yourself with the periodic table, as it provides a roadmap for predicting common oxidation numbers of elements.
- Practice, Practice, Practice: Working through examples and problems will solidify your understanding and build confidence in deciphering oxidation numbers.
- Visualize the Process: Draw diagrams or use online simulations to visualize electron transfer during redox reactions, making the concepts more tangible.
- Seek Guidance: Don’t hesitate to consult textbooks, online resources, or your instructors if you encounter challenges. Remember, learning is a collaborative process, and asking questions is a sign of strength, not weakness.
Oxidation Number Calculator
Embarking on a Journey of Chemical Discovery
As you’ve journeyed through this guide, you’ve unlocked the secret language of oxidation numbers. You’ve learned how to decipher the code using oxidation number calculators and gained a comprehensive understanding of the concept’s significance. Oxidation numbers are not just abstract numbers; they are the key to unlocking a world of chemical possibilities, guiding us through the fascinating realm of chemical reactions. So, grab your oxidation number calculator, experiment with different compounds, and embark on a journey of chemical discovery. The world of atoms awaits your exploration! Remember, you can share your insights, ask questions, or simply explore further resources, continuing your journey into the wonderful world of chemistry.






