Have you ever wondered how scientists can identify the hidden secrets within the molecular world? It’s like having a magical microscope that can reveal the intricate dance of atoms within a molecule. This magic, in essence, is infrared spectroscopy, a powerful technique that allows us to “see” the invisible by probing the vibrational frequencies of molecules. And when it comes to carboxylic acids, this technique becomes an invaluable tool, painting a vivid picture of their chemical nature and allowing us to unravel their unique characteristics.
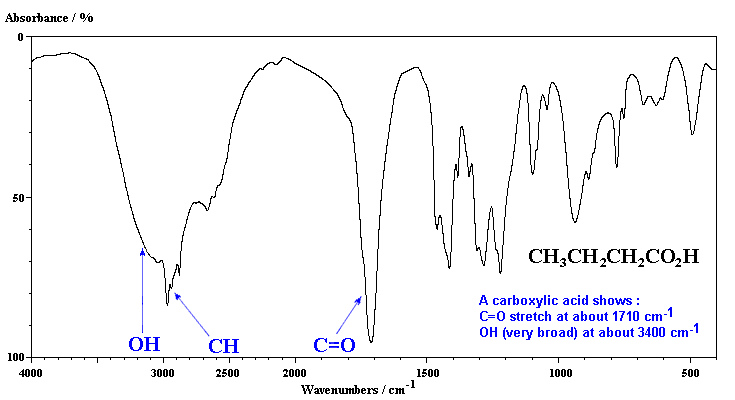
Image: www.chem.ucalgary.ca
Carboxylic acids, with their signature -COOH functional group, are ubiquitous in nature and hold significant importance in various fields. From the tangy flavor of vinegar to the complex processes of life, carboxylic acids play a crucial role in our world. But how do we understand their properties and behavior? This is where infrared spectroscopy steps in, providing a detailed fingerprint that uniquely identifies each carboxylic acid and allows us to delve into their chemical structures and functionalities.
Unveiling the Vibrational Symphony of Carboxylic Acids
Imagine a molecule as a tiny orchestra, each atom playing its own vibrational note. Infrared spectroscopy allows us to listen to this symphony, revealing the characteristic frequencies at which each molecule vibrates. For carboxylic acids, these vibrations are particularly intriguing.
The -COOH group, the heart of every carboxylic acid, contributes its unique set of vibrations:
- C=O Stretch: This is the strong, sharp band around 1700 cm-1, a telltale sign of the carbonyl group. Its frequency can vary slightly depending on the nature of the molecule, giving us clues about the electronic environment surrounding the carbonyl group.
- O-H Stretch: This broad band, typically appearing between 2500 and 3300 cm-1, reflects the hydrogen bonding interactions between carboxylic acid molecules. The strength and width of this band can provide insight into the degree of hydrogen bonding present.
- C-O Stretch: This band, usually observed around 1200 cm-1, represents the stretching vibration of the carbon-oxygen single bond within the carboxylic acid group.
- O-H Bend: This band, appearing around 900 cm-1, reflects the bending motion of the hydroxyl group.
These vibrational frequencies act as a fingerprint, uniquely defining each carboxylic acid. By analyzing the infrared spectrum, we can identify the presence of carboxylic acids, differentiate between different types of carboxylic acids, and even gather valuable information about their structure and properties.
Beyond the Fingerprints: Applications of Carboxylic Acid IR Spectroscopy
The insights gained from infrared spectroscopy extend far beyond simply identifying carboxylic acids. It’s a powerful tool with wide-ranging applications:
- Material Science: Infrared spectroscopy helps characterize polymers, plastics, and other materials containing carboxylic acids, leading to the development of innovative materials with specific properties.
- Environmental Monitoring: This technique is crucial in monitoring air quality, identifying potential pollutants, and analyzing the presence of carboxylic acids in various environmental samples.
- Pharmaceutical Research: Carboxylic acids are ubiquitous in pharmaceuticals. Infrared spectroscopy plays a critical role in developing new drugs, ensuring their purity, and monitoring their stability.
- Food Chemistry: Infrared spectroscopy helps identify the presence and quantity of carboxylic acids in food products, ensuring quality control, and understanding their role in flavor and preservation.
Expert Insights and Actionable Tips
Dr. Emily Carter, a renowned chemist specializing in infrared spectroscopy, highlights the importance of understanding the nuances of infrared spectra: “While the basic principles of carboxylic acid analysis are straightforward, the interpretations can be complex. Considering factors like peak intensity, peak shape, and the presence of other functional groups is essential for accurate analysis.”
For those beginning their journey in the world of infrared spectroscopy, here are some practical tips:
- Start with a simple database: Familiarize yourself with common carboxylic acid IR spectra as a reference point.
- Focus on the key bands: Pay attention to the C=O stretch, O-H stretch, and C-O stretch frequencies, as they provide the most telltale signs of carboxylic acids.
- Analyze the peak shapes: Pay attention to the broadness and sharpness of peaks, as they can reveal valuable information about hydrogen bonding and molecular interactions.
Image: www.chemguide.co.uk
Carboxylic Acid Ir
https://youtube.com/watch?v=8RL-Ir0bfS8
Conclusion
Infrared spectroscopy is an invaluable tool for understanding the intricate world of carboxylic acids. From identifying their presence to discerning their unique properties, this technique empowers us to gain a deeper understanding of these fundamental molecules. As we continue to unravel the intricacies of carboxylic acids, infrared spectroscopy stands as a vital tool in advancing our scientific knowledge and its countless applications. So next time you see a flask of vinegar or a pill in your medicine cabinet, remember that hidden within these simple compounds lies an intricate story revealed through the silent language of molecular vibrations.






