Have you ever wondered how seemingly simple compounds like baking soda (sodium bicarbonate) or the ammonia used to clean your home are actually complex assemblies of atoms? At the heart of these everyday molecules lies a fascinating world of polyatomic ions — groups of atoms bonded together that carry a charge. Understanding these “building blocks” is essential for comprehending countless chemical reactions, from the processes that fuel our bodies to the synthesis of life-saving medications. This journey into the polyatomic ions chart will equip you with the knowledge to decipher the intricate language of chemistry.
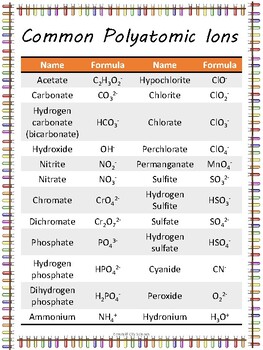
Image: www.teacherspayteachers.com
Imagine staring at a complex chemical formula, filled with symbols and subscripts. Initially, it can appear daunting, but with the right tools, it becomes a roadmap to understanding the composition and behavior of matter. The polyatomic ions chart acts as your guide, unveiling the hidden intricacies of these charged entities. It’s a window into the fundamental building blocks that make up the vast array of compounds around us, from the minerals in our soil to the intricate molecules found in our DNA.
Delving Deep: The World of Polyatomic Ions
Let’s embark on a journey to decipher the mysteries of polyatomic ions! These fascinating entities are groups of two or more atoms bonded together, forming a unit with a net positive or negative charge. Here’s where their unique nature shines: while individual atoms can gain or lose electrons, becoming simple ions like chloride (Cl-) or sodium (Na+), polyatomic ions possess an overall charge distributed across the entire group.
Think of a polyatomic ion like a team of atoms working together, pooling their electrons to achieve stability and create a distinct entity. A great example is the nitrate ion (NO3-). This group consists of one nitrogen atom and three oxygen atoms, all tightly bonded. The entire group carries a negative charge, making it a crucial component in a wide array of chemical reactions.
Now, let’s explore the intriguing world of polyatomic ions by examining their key characteristics and exploring their diverse roles in chemistry:
1. The Building Blocks of Complexity
Polyatomic ions are the superheroes of chemical reactions. They act as crucial building blocks for countless compounds, shaping the world around us. Imagine the humble baking soda we use every day. It’s chemically known as sodium bicarbonate (NaHCO3), and its formation depends on the crucial presence of the bicarbonate ion (HCO3-). This ion, comprising a single carbon atom, one hydrogen atom, and three oxygen atoms, is responsible for the unique properties of baking soda, making it a cornerstone of baking and a versatile ingredient in countless household products.
2. A Gateway to Understanding Chemical Reactions
Polyatomic ions are the key players in countless chemical reactions. They participate in the synthesis, decomposition, and transformation of molecules, driving countless processes crucial to life and technology. For instance, the phosphate ion (PO43-) is a fundamental component of DNA, the blueprint of life, and it also plays a critical role in energy transfer within our cells. Understanding the behavior and interactions of these polyatomic ions is fundamental to unraveling the complex dance of chemical reactions that govern our world.

Image: www.pinterest.es
3. A Glimpse into the Diversity of Nature
The polyatomic ions chart offers a captivating glimpse into the diversity that exists in nature. It reveals how atoms can combine in various ways to form an array of charged entities, each with unique properties and functions. From the sulfate ion (SO42-), responsible for the sourness of vinegar, to the carbonate ion (CO32-), present in limestone and seashells, these ions showcase the remarkable versatility of chemical bonds in shaping the world we inhabit.
4. A Guide to Navigating Chemical Formulas
The polyatomic ions chart is an invaluable tool for decoding the seemingly cryptic language of chemical formulas. By understanding the common polyatomic ions and their charges, deciphering complex formulas becomes a breeze. For instance, when you encounter the formula CaSO4 (calcium sulfate), the chart reveals that the sulfate ion (SO42-) carries a negative charge, which balances the positive charge of the calcium ion (Ca2+). It’s like having a cheat sheet for unraveling the secrets of chemical composition.
Navigating the Polyatomic Ions Chart: A Practical Guide
The polyatomic ions chart may seem dense at first glance, but it’s designed for clarity and ease of use. Here’s how to navigate it effectively:
- Identify the ion’s name: Each polyatomic ion has a distinct name. For example, you’ll find entries like “carbonate” or “nitrate” on the chart.
- Locate its chemical formula: Alongside each name, you’ll see the corresponding chemical formula, which represents the ion’s composition.
- Focus on the charge: The chart clearly indicates the charge of each polyatomic ion, which is crucial for understanding its behavior in chemical reactions.
Harnessing the Power of Polyatomic Ions: A Practical Perspective
The polyatomic ions chart is more than just a table of information; it’s a gateway to understanding the fundamental building blocks of chemical reactions and the intricate processes that shape our world. Here are some practical applications of this knowledge:
- Everyday Chemistry: From the baking soda in your kitchen to the cleaning products in your bathroom, polyatomic ions are ubiquitous in our daily lives. By understanding these ions, you can grasp the chemical reactions behind these common products and make informed choices.
- Medical Advancements: The study of polyatomic ions plays a vital role in understanding how our bodies function and developing new medications. By understanding how these ions interact, we can create more effective treatments for diseases.
- Environmental Sustainability: Polyatomic ions are crucial in understanding environmental processes and addressing pollution issues. By studying their behavior, we can develop solutions for cleaner energy production and sustainable practices.
Polyatomic Ions Chart
Conclusion: The Enduring Significance of Polyatomic Ions
The polyatomic ions chart is a treasure trove of knowledge, offering a fascinating window into the building blocks of countless compounds and chemical reactions. By deciphering its secrets, we gain a deeper understanding of the world around us – from the processes that fuel our bodies to the technologies that shape our future. So, the next time you encounter a seemingly complex chemical formula, remember the polyatomic ions chart, your key to unlocking the intricate language of chemistry and revealing the hidden wonders of the molecular world!






