Ever noticed how a perfectly ripe banana turns brown after sitting on your counter for a few days? Or how the vibrant green leaves of a tree transform into brilliant shades of red and yellow in autumn? These familiar changes in color might seem simple, but they actually tell a complex story about the science behind matter and its transformations. Today, we’re going to explore the fascinating world of chemical changes, specifically focusing on the question: is a change in color always a sign of a chemical reaction? Let’s dive in!

Image: sciencenotes.org
This question truly piqued my curiosity when I was a young child, fascinated by the way a simple glass of lemon juice could magically change the color of a pink flower. Was this a result of a chemical reaction? Or was it simply a matter of mixing, like the way you might swirl blue and yellow paints together to create green? I soon learned that understanding the relationship between color change and chemical reactions was far more intricate than I initially imagined.
Understanding The Difference Between Physical And Chemical Changes
To unravel the mystery behind color change and its connection to chemical change, we need to first establish a clear understanding of the difference between physical and chemical changes. A physical change alters the appearance or form of a substance without changing its chemical composition. Think about melting ice – it changes from a solid to a liquid, but it’s still water (H2O). On the other hand, a chemical change involves the creation of a new substance with a different chemical composition. Think about burning wood – the wood transforms into ash, carbon dioxide, and other products, altering its chemical structure.
Now, let’s delve deeper into the intriguing relationship between color and chemical change. While a change in color can indicate a chemical reaction, it’s not always the case. The color of a substance is determined by how its molecules absorb and reflect light. In some physical changes, the molecules rearrange themselves, altering their ability to absorb and reflect light, leading to a color change. This is a common phenomenon in materials like the aforementioned ripe banana. The banana doesn’t change its chemical composition when it browns, but rather the structure of its molecules changes, leading to the color change.
Chemical Reactions And Color Change
On the other hand, when a chemical reaction occurs, the molecules of the original substance break apart and recombine to form new molecules. This process often involves changes in the way these new molecules absorb and reflect light, leading to a change in color.
For example, when you drop an iron nail into a solution of copper sulfate, the iron reacts with the copper sulfate to create copper metal, which has a distinct reddish-brown color. This change in color visibly demonstrates a chemical reaction taking place.
Another classic example is the reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid). The resulting reaction produces carbon dioxide gas (CO2), which is colorless and odorless, but also a change in color from the original clear vinegar to a cloudy white mixture, indicating a chemical change.
Why Color Change Can Be Misleading
It’s crucial to remember that color change alone isn’t a definitive indicator of a chemical reaction. There are instances where color change can occur without any chemical transformation. For example, mixing blue and yellow food coloring creates green color – a change in perception but not a chemical change.
Furthermore, some physical changes can result in color changes that might initially appear like chemical reactions. For example, dissolving a colored salt in water can change the color of the solution, but this is a physical change, as the salt molecules are simply dispersing in the water. It’s crucial to consider the context and other accompanying changes to determine whether a change in color truly indicates a chemical reaction.
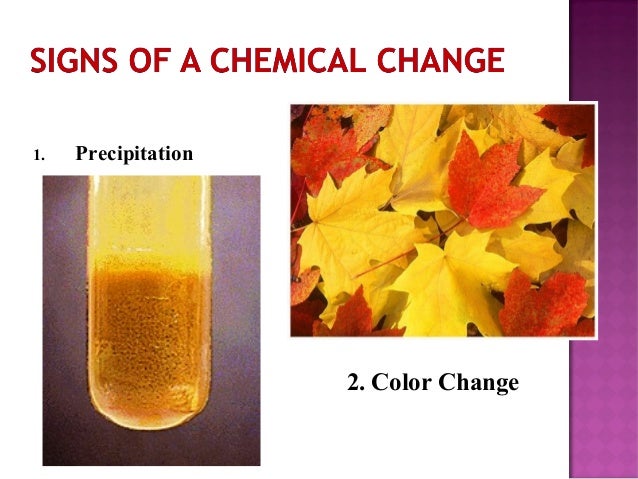
Image: www.slideshare.net
Examples of Color Changes in Everyday Life
The real world abounds in examples of color change, both those associated with chemical reactions and those without. Let’s explore a few:
- Leaves changing colors in autumn: This change is primarily due to the breakdown of chlorophyll, a green pigment in leaves. As temperatures drop and days become shorter, chlorophyll production decreases, allowing other pigments like carotenoids (yellow and orange) and anthocyanins (red and purple) to become visible.
- Rusting of iron: When iron reacts with oxygen in the presence of moisture, it forms iron oxide, commonly known as rust. This change in color from silvery iron to reddish-brown rust signifies a chemical change.
- Cooking an egg: The clear, watery egg white turns opaque white when heated, indicating a chemical change as proteins in the egg denature and coagulate.
- Burning a candle: The wax melts and burns, transforming into carbon dioxide and water. The flame itself results from the chemical reaction of combustion, showcasing a change in color.
- Mixing solutions: Combining clear vinegar and red cabbage juice results in a vibrant purple solution. The change in color is due to the interaction of the pigments in the cabbage juice with the acid in the vinegar.
Expert Tips for Understanding Color Changes
If you’re curious about whether a specific color change involves a chemical reaction, here are a few tips to help you determine its nature:
-
Look for other evidence of chemical change: If you see a change in color, consider other factors like gas bubbles, temperature change, or a change in smell. These all potentially indicate a chemical reaction.
-
Consider the context: Think about the situation and the substances involved. Are you mixing chemicals, heating something, or exposing it to a different environment? The context can provide valuable clues.
-
Research the substances: If you’re not sure about the chemical composition of the substances involved, research them online or consult a textbook to understand their potential reactions.
-
Conduct careful experiments: When experimenting with color changes, start with small amounts and observe carefully. You may want to use a control group (an unchanged sample) for comparison.
FAQ About Color Change and Chemical Change
Here are some frequently asked questions about color changes and chemical changes:
Q: Is all color change permanent?
A: No, some color changes are temporary, such as the change in color of leaves in autumn. Other color changes, like rusting, are permanent. The permanence of the color change depends on the nature of the change (physical or chemical) and the stability of the new substance formed.
Q: Can color change be used to identify chemical reactions?
A: While color change can be a helpful indicator, it’s not a foolproof method. Other changes, like the formation of gas bubbles, a change in smell, or the release of heat or light, are also important indicators of chemical reactions.
Q: Are there any tools that can help me determine if a color change is due to a chemical reaction?
A: Yes, there are several tools that can help. Spectrophotometers, for example, measure the amount of light absorbed and transmitted by a substance at different wavelengths, providing a more detailed analysis of color changes and the possibility of chemical reactions.
Is Change In Color A Chemical Change
Conclusion
Understanding the relationship between color change and chemical changes is a fascinating journey into the world of chemistry. While a change in color can sometimes be a reliable indication of a chemical reaction, it’s crucial to remember that it’s not always the case. By carefully considering the context, observing for other changes, and conducting thorough research, you can unravel the mysteries behind these captivating color transformations.
Are you interested in delving deeper into the world of chemical reactions and their impact on color change? Let me know in the comments below, and we can explore more examples and experiment ideas together!






